
Written by: Brett Mackay, BS
KEY TAKEAWAYS
- Feed analysis optimizes rations, boosts efficiency, and supports sustainability.
- Wet chemistry is the gold standard for precise nutrient data.
- NIR is fast, low-cost, and reliable when well calibrated.
- In situ and in vitro show real digestion and fermentaion.
- Combined methods improve accuracy and nutrient use.
Nutrient Analysis: Why do we Care?
While the breadth of feed sample analysis methodology can feel daunting, understanding the nutrient quality and digestibility of feeds fed to dairy cattle is a key component of dairy nutrition. Determining the nutrient content of feeds allows for their utilization to be optimized, rumen fermentation to be enhanced, and financial returns to be maximized, while limiting the excretion of unused nutrients into the environment. A clear understanding of the concentration and availability of nutrients in each feed ingredient is critical for achieving nutrient balance to meet a cow’s protein and energy requirements, along with predicting the speed of digestion. Today, chemical analysis, near-infrared reflectance spectroscopy (NIR), in situ analysis, and in vitro gas fermentation are the primary tools for evaluating nutrient concentration and availability of feeds.
Wet Chemistry
Chemical analysis, commonly called “wet chemistry”, relies on laboratory tests that measure protein, fiber, starch, and fat. Energy values can then be estimated with mathematical equations. The wet chem methodology behind several core nutrients is outlined below:
Crude Protein
The most common method of estimating the protein content of feeds is through the Kjeldahl method, which quantifies total nitrogen in feed by converting nitrogen compounds into ammonia. Multiplying the nitrogen value by 6.25 (because proteins contain about 16% nitrogen by weight) provides crude protein content.
Fiber
To determine a feed’s fiber content, the detergent system developed by Van Soest et al. is used. Neutral detergent dissolves soluble fiber, leaving neutral detergent fiber (NDF). Acid detergent removes hemicellulose, leaving cellulose and lignin, measured as acid detergent fiber (ADF).
Starch
Starch concentration can be determined using the enzymatic-colorimetric starch assay, which converts starch into glucose. The intensity of the color reaction reflects glucose concentration, which is then multiplied by 0.9 (because of a water molecule that is lost when glucose is linked together) to determine starch content.
Fat
Ether extract (EE) measures crude fat by dissolving lipids such as galactolipids, triglycerides, and phospholipids. The solvent is removed through pressure, evaporation, or filtration, and the residue is weighed. The difference in weight before and after extraction indicates fat content.
Wet chemistry is highly accurate for defining nutrient quality and digestibility, but it is time-consuming, requires skilled labor, and generates chemical waste.
Near-Infrared Reflectance-Spectroscopy (NIR)
NIR uses infrared light to characterize nutrient concentration in feeds and is the fastest and most inexpensive way to analyze a feed. Organic matter absorbs light at specific wavelengths depending on the chemical bonds present. These absorption patterns create unique spectra for each feed ingredient when “scanned”. When calibrated against wet chemistry results, NIR provides a rapid, cost-effective, and reliable method for assessing a wide variety of feeds. However, accuracy is reduced with complex samples such as total mixed rations (TMR), where particle size and ingredient diversity complicate predictions. NIR therefore depends on robust calibration and cannot fully replace wet chemistry as it is an indirect measure of the nutrients in a sample
In Situ Analysis

The image shows a fistulated cow that allows researchers to study rumen digestion firsthand — helping refine feed analysis methods and improve how nutrients are utilized for healthier, more efficient dairy herds.
While wet chemistry and NIR define nutrient content, they are disconnected from biological digestion and nutrient availability. In situ analysis links feed disappearance to actual digestion within the cow. Ground feed samples are placed in small porous bags (so bacteria can pass through, but the feed can’t escape), grouped into a larger weighted nylon bag, and inserted into the rumen, duodenum, or ileum of a cannulated cow. After incubation, the bags are removed, washed, and dried before testing for dry matter, protein, NDF, and starch disappearance. This method has been critical in identifying the rate, site, and extent of digestion, each of which are essential for dynamic feed formulation models. However, it is invasive, limited to research and teaching herds, and impractical for broad commercial use. In addition, in situ techniques do not account for the natural chewing and rumination process of the cow. Samples are ground prior to incubation, which exposes greater surface area to microbial attack. As a result, feeds with inherently poor physical digestibility may appear slightly more digestible in the laboratory than in practice, since mastication, particle size reduction, and passage rate all affect digestion in the live animal. For this reason, nutritionists often use tools to assess the physical characteristics of a feed, such as a Penn State Particle Separator, which in combination with lab analyses provide additional insight into a feed’s actual digestive kinetics.
In Vitro Gas Fermentation
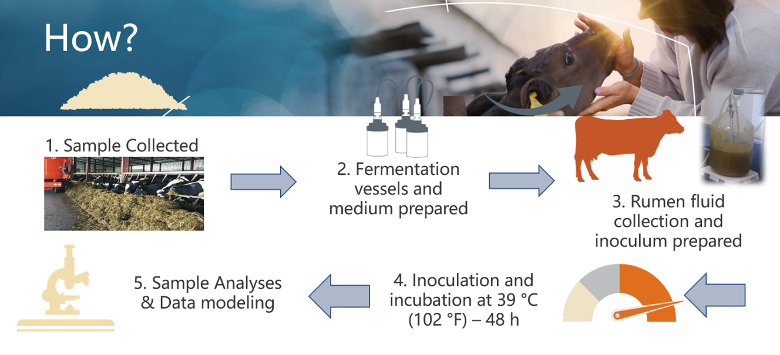
In vitro gas fermentation provides an alternative approach by simulating rumen fermentation in the laboratory. Feed samples are incubated in sealed vessels containing rumen fluid, and gas production is continuously monitored as an indicator of microbial metabolism. Compared to fixed-point disappearance methods (like in situ discussed above), gas curves capture the dynamics of digestion over time. Gas production patterns are closely associated with volatile fatty acid (VFA) profiles: acetate and butyrate production generate more gas, while propionate yields less. Digestion is typically modeled as a dual pool system, with rapidly fermentable carbohydrates such as sugars, high-moisture corn, flaked corn, and byproducts in the fast pool, and slower-digesting starches and digestible NDF in the slow pool. The fast pool is associated with higher propionate production in the rumen, while the slow pool yields acetate and butyrate. Protein and fat produce relatively little gas and are usually excluded from these models. In vitro fermentation allows researchers to estimate lag time before gas production ramps up, degradation rates, and total digestibility, offering practical insights into how individual feeds or TMRs are digested. However, like in situ methods, in vitro techniques rely on ground feed substrates rather than chewed material. This pre-processing can enhance microbial access and artificially improve digestibility values compared to what would occur in the rumen. Thus, while in vitro gas production models are highly useful for comparing digestion kinetics, they do not fully replicate the effects of particle size, chewing activity, or rumination on feed utilization.

Results from the Alltech IFM® (In Vitro Fermentation Model); the top panel illustrates gas production over time for fast, slow, and total nutrient pools relative to reference values. The bottom panel tabulates data related to individual nutrient pools, digestibility, and microbial production, with corresponding color scales highlighting results “low” to “high” compared to typical lab values.
Better Together
Wet chemistry and NIR provide accurate nutrient concentrations and remain essential for ration formulation, with wet chemistry serving as the calibration standard. In situ analysis plays a vital role in research, advancing understanding of digestion kinetics and informing dynamic feed models. In vitro gas fermentation extends this knowledge by characterizing digestion rates and partitioning substrates into fast and slow pools. Together, these approaches complement one another: wet chemistry and NIR quantify feed composition, while in situ and in vitro techniques reveal biological availability and fermentation dynamics. Integrating these tools enhances the precision of ration formulation, improves nutrient efficiency, and supports both economic viability and environmental sustainability. Looking forward, continued development in predictive modeling, machine learning calibration, and precision feeding systems will help bridge laboratory analyses with on-farm decision making.
Standard Dairy Consultants can help.
We understand you measure our value by the results our people deliver. Our experienced team knows how to apply their knowledge to your specific operation in ways that directly impact your efficiency and performance and, ultimately, your profitability. The only way to be good at what we do is to develop a good working relationship with you. We’re regular fixtures at your farm as we get to know you, your people and your herd.


